Periodicity & trends 1.2
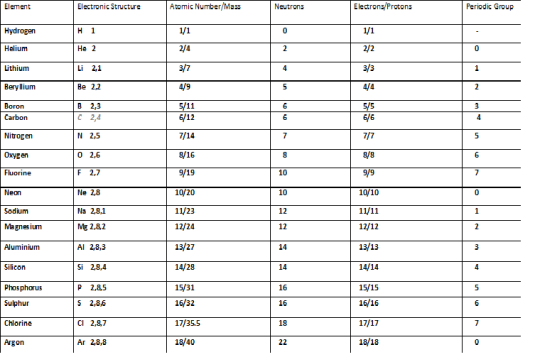
Task 1- Using the periodic table, describe the atomic structure of the elements from 1 -20. [P3]
*Click table to increase size
Task 2 – Describe how the atomic structure of the elements mentioned in Task 1 differ. Think about whether they have the same shell number, the same electrons. [M3]
The elements described in Task 1 have different atomic structures and properties based on their periodic group, also their group number correlates with the numbers of electrons in their last shell, providing that they have a periodic group.
As demonstrated above in Task 1, elements with an electronic shell ending in X number have a periodic group that matches said number, with full shells equalling the group o and any number from 1 to 7 equalling a matching group number.
The number of protons to electrons as demonstrated above is always the same for each element while their numbers of neutrons differs based on a simple formula:
AM (Atomic Mass) – AN (Atomic Number) = Number of neutrons
As you go down each periodic group, although they retain their common properties, the elements change. All elements in fact have more shells as you go down in their respective periods, this is because the numbers of shells an electron has is related to its atomic number and elements are classified by atomic number, so naturally the ones with the biggest atomic numbers are found at the bottom of the table.
Task 3 – Compare the atomic structure of groups 1 & 7 and describe any patterns in their properties.
*Click table to increase size
Patterns in their properties:
| Properties | Group 1 | Group 7 |
| State | All shiny soft metals | Changes from gas – liquid – solid as the atomic number increases |
| Reactivity | Increases the higher the atomic number is | Decreases as you go down the group |
| Melting & Boiling point | Decreases the higher the atomic number is | Increases the higher the atomic number is |
| Densities | Increases the higher the atomic mass is | Increases the higher the atomic mass is |
Task 4 – You are to conduct an experiment on the elements from group 1 & 7. Write up the practical showing suitable results and conclusion. [P4]
Experiment 1 – Reactions of iodine with zinc
The aim of the experiment was to study the reactions of halogen elements, when in contact with other chemicals, in this study, iodine & zinc.
Hypothesis
- Iodine when in contact with zinc will form a salt.
Method – The experiment was set up as such:
- 4 cm³ of alcohol (ethanol) was added to a test tube.
- Iodine crystals were dissolved in the alcohol.
- Powdered zinc was added to the solution.
- The liquid was stirred.
- Observation of any chemical reaction which had taken place.
- The liquid was filtered into another test tube to filter out any excess zinc.
- The filtered liquid was deposited onto a watch glass sitting on top of beaker filled with hot water.
- Observation of the liquid.
Results
- Iodine when dissolved in ethanol becomes a dark brown colour. When zinc is added, the temperature of the solution increases, indicating an exothermic reaction. Once the reaction is over the dark brown colour of the iodine fades and the excess zinc is left out. The solution after being filtered and made to evaporate using a watch glass and beaker filled with hot water leaves out zinc iodide.
Conclusion
- When iodine dissolved in ethanol comes in contact with zinc, an exothermic reaction occurs, this reaction forms zinc iodide – a metal salt which can be harnessed by evaporating the solution.
Experiment 2 – Reactions of halogen solutions on universal indicator paper
The aim of the experiment was to study the reactions of halogen solutions on Universal Indicator paper.
Method – The experiment was set up as such:
- Universal Indicator paper was placed on a white tile.
- 3 halogens solutions (bromine, iodine, chloride) were tested separately on the Universal Indicator paper.
- Each reaction with the paper was observed and noted.
Results – The halogen solutions react with the universal paper, these are the results that were observed:
| Universal Indicator paper | |
| Chlorine water | Turns red then quickly bleaches white |
| Bromine water | Turns red then slowly bleaches white |
| Iodine water | Brown stain |
Conclusion
- Chlorine bleaches the entire indicator paper; bromine only bleaches the area on which it was applied and iodine faintly bleaches the spot where it was applied. All 3 halogens react with water to produce a strong and weak acid which has bleaching properties and is an oxidising agent. The extent of the reaction decreases in the same way that the reactivity of halogens do as their atomic number increases, this is observed and proven to be true with the results of the test performed above in experiment 2.
Experiment 3 – Reactions of halogens with chemicals
The aim of the experiment was to study the reactions of halogen elements when in contact with other chemicals, in this study, potassium, bromine, iodine and chlorine.
Hypothesis
- Halogens will displace weaker chemicals from solutions, in this case the weaker halogens will be displaced by the stronger ones, e.g. chloride displaces bromine.
Method
- The experiment was set up as such:
- Halogen solutions were each put in separate test tubes.
- Each halogen solution was separately mixed with a solution of potassium + bromine or iodine or chlorine.
- Any reaction was observed and noted.
Results – The halogen solutions react with the potassium + halogen solutions and displacement reactions occur:
| Potassium chloride solution | Potassium bromide solution | Potassium iodide solution | |
| Chlorine water | No reaction | Yellow/orange colour (bromine) | Brown colour (iodine) |
| Bromine water | No reaction | No reaction | Colour changes from yellow/orange/brown |
| Iodine water | No reaction | No reaction | No reaction |
Conclusion
- Reactivity of the halogen elements decrease as their atomic number increases and displacement reaction occur when in contact with other halogens. The experiment confirms this with chlorine displacing both bromine and iodine from the solutions. The reactivity of iodine is so small that it was not detected in the experiment. Therefore, according to these properties and confirmed by the experiment, the order of reactivity of the halogens used in this experiment is as follows: chlorine à bromine à iodine.
Task 5 – Explain why the elements in groups 1 and 7 are mainly used to form compounds. [M4]
Alkali metals are ideal to form compounds with other elements because like all atoms they want to get rid of any electrons that aren’t part of a full outer shell, however unlike all atoms they only have 1 electron in their outer shell which makes them more reactive then other elements, as the closer electrons are to having a full outer shell the more reactive they are.
Furthermore they all react vigorously with water, creating heat and even explosions depending on the element in question upon contact with water, for that particular reason they are kept in mineral oil as to avoid any contact with water, air or anything that could cause them to react with.
Halogens are very useful in the formation of compounds because of their characteristic, such as displacing other less reactive halogens in compounds e.g. removing iodine from potassium iodine using chloride to make potassium chloride and having iodine separate itself. They also react aggressively with most elements.
Group 1 and 7 are both highly reactive because they both only require 1 more or less electron to have a full outer shell which creates very aggressive reactions when they come into contact with each other or most other elements. A property they have when combined is the ability to form ionic salts. These salts are the result of neutralization; this occurs because when both groups react together they form full outer shells, resulting in the formation of a neutral compound.
It is because they react so much to other elements that they are mainly used to form compounds, as it is much easier with them than most other elements.
Task 6 – Explain the pattern in chemical behaviours of group 1 and 7 including their electronic structure. [D2]
Reactivity
- Both groups are highly reactive due to being so close to having a full outer shell.
Group 1 becomes more reactive as you go down the group; this is because the further down you go the more shells the elements have. The pull exercised by the atom’s nucleus is weaker and more easily broken the further apart the electrons are from it. As such, an element’s reactivity can be guessed using its electronic configuration.
Alkali metals have all 1 electrons in their outer shell, for metals the fewer electrons they have in their outer shell the more reactive they are as it takes less energy to get rid of 1 electron than to get rid of 2 or more. Now the further down the group 1 you go, the more shells the elements will have and the weaker the pull from the nucleus will be. As a general rule metal elements such as group 1 tend to want to give over their electrons in the outer shell to non metal elements when they react together.
Group 7 becomes less reactive as you go down the group, but for the same reasons as metal elements, the only thing that changes is the rule under which they operate. Halogens & most non metal elements have opposite rules of metal elements. The more electrons they have in their outer
shell, the more reactive they become. This is because as a general rule, non metal elements tend to want to complete their incomplete outer shell rather than get rid of it like metal elements. Following this same rule, the more shells they have the less reactive they become, because the nucleus’s pull on the electrons is weaker and requires more force.
Melting and boiling point
The melting and boiling point of group 1 decrease as their atomic number increases because they have more shells. The energy needed to overcome the metal bonds decreases the more shells their atoms have because the force holding the metal ions is weaker due to the nucleus being so far from the electrons.
For group 7 the melting and boiling point decreases as their atomic number increases. Unlike metals, melting/boiling a halogen involves breaking intermolecular forces (because halogens have diatomic molecules), these forces are in between the molecules and increase in strength as the size of the molecule increases as you go down the group, it takes more energy to break these forces and so it needs a higher melting/boiling point.
In conclusion
For metal elements, in this case the focus being alkali metals:
- The fewer electrons in the outer shell, the more reactive they will be. In the same groups, elements will have the same amount of electrons in their outer shell so the reactivity will be decided based on the number of shells they have.
- The more shells they have, the weaker the pull from nucleus will be and the greater their reactivity will be.
- The reactivity of metal elements will increase, based on the number of electrons in their outer shell and the number of shells they have, all of which are revealed by the electronic structure.
- The more shells metal elements have, the less energy is needed to melt/boil them.
For non metals elements, in this case the focus being halogens:
- The more electrons in the outer shell, the more reactive they will be. In the same groups, elements will have the same amount of electrons in their outer shell so the reactivity will be decided based on the number of shells they have.
- The fewer shells they have, the stronger the pull from the nucleus will be and the greater their reactivity will be.
- The reactivity of non metal elements will increase, based on the number of electrons in their outer shell and the number of shells they have, all of which are revealed by the electronic structure.
- The more shells a non metal element has, the more energy is needed to melt/boil them.